Abstract
Background: Venous thromboembolism (VTE) is a recognized complication of sickle cell disease (SCD). Several studies confirm that SCD itself is an independent risk factor for development of VTE. However, the optimal pharmacologic anticoagulant remains unknown.
Methods: This retrospective single-institution cohort study was exempt by the Institutional Review Board. Data were collected via review of electronic medical records including ambulatory, emergency department, general floor, and intensive care unit encounters. Patients with SCD were identified spanning 1/2009-7/2017 using ICD 9/10 codes. Inclusion criteria were age ≥18 years at time of VTE diagnosis, imaging confirming VTE, and documented compliance based on INR values and/or provider/pharmacy documentation. VTE diagnosis included deep vein thrombosis (DVT) at any location and pulmonary embolism based on documented imaging and ICD 9/10 codes. Anticoagulants included direct oral anticoagulants (DOACs), vitamin K antagonists (VKA), and low-molecular-weight heparin (LMWH). The DOACs used in this study were rivaroxaban, apixaban and dabigatran. Exclusion criteria were known active malignancy, confirmed hypercoagulable risk factors beyond SCD, atrial fibrillation and/or history of major bleeding prior to anticoagulation. Due to low event rates, a log likelihood ratio test of independence was calculated for associations between drug type and two endpoints: bleeding rate and rate of VTE recurrence. Rate of VTE recurrence was defined as a newly diagnosed VTE within 6 months of initiation of anticoagulation. Bleeding rate was defined using International Society on Thrombosis and Hemostasis criteria: bleeding event into a critical site and/or a ≥2 point decrease in baseline hemoglobin.
Results: A total of 109 patients with SCD met inclusion criteria. 66 patients (60%) were female. SCD genotypes represented included HbSS in 91 patients (83%), HbSC in 12 (11%) and HbS β+ thalassemia in 4 (4%). There were no patients with HbS-β0 thalassemia. VTEs consisted of 69 DVTs and 43 pulmonary emboli. 31 out of 109 VTEs were provoked, including 30 catheter-related incidents. After initial VTE event, 32 patients received a VKA, 34 received LMWH, and 43 received a DOAC. Within the class of DOACs, 31 patients received rivaroxaban, 5 received apixaban, and 7 received dabigatran.
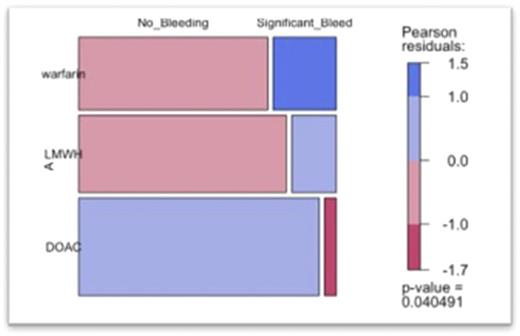
Sixteen of 109 patients (15%) experienced a clinically significant bleeding event, including 8 on VKA, 6 on LMWH, and 2 on a DOAC. Bleeding incidence was least with the DOAC class [0.22 CI (0.04-0.84) p < 0.05], greatest with warfarin [1.55 CI (0.57-4.33) p < 0.05] and slightly less with LMWH [0.64 CI (0.23-1.73) p < 0.05]. There was a significant decrease in incidence of bleeding events in patients receiving a DOAC for anticoagulation, compared to a VKA or LMWH (p = 0.033).
At a median follow-up of 11.8 months (range, 3.4 - 60 months), 33 patients had a recurrent VTE, including 10 on VKA, 10 on LMWH and 13 taking a DOAC (p = 0.833). An association between VTE and SCD genotype could not be identified due to small numbers of patients with non-HbSS genotypes.
Conclusion: In patients with SCD and VTE, there was a significant decrease in incidence of bleeding events in patients receiving a DOAC for anticoagulation, compared to a VKA or LMWH (p = 0.033). There was no difference between VTE recurrence rate and choice of initial anticoagulation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal